"Precision Cleaning - The Magazine of Critical Cleaning Technology"
Parts Cleaning
Ultrasonic Cavitations and Precision Cleaning
by: Sami B Awad
Pages: 12-17; November, 1996
Precision or critical cleaning is currently in great demand and is expected to increase in the future. The rapid advancements in various current technologies and the constant trend in miniaturizing of components have created a need for higher cleanliness levels. Contamination in the level of monolayers can drastically alter surface properties such as wettability, adhesion, optical or electrical characteristics. Particles in the range of few microns down to submicron levels, trace contaminants such as non-volatile residues (NVR) in the range of micrograms/cm2 and pictogram/cm2, ionics in the same range or traces of corrosion have become part of the daily concerns of the manufacturing engineers in major industries such as semiconductors, automotive, disk drive, optics, ophthalmic, glass, medical, aerospace, pharmaceuticals and tool coatings, among others.
The specifications on trace contaminants and particle sizes are being tightened periodically to reflect the new technology trends. Every industry has its own set of cleanliness specifications and the focus differs. For example, while NVR has not been an automotive industry issue until now, it has been crucial for the semiconductor and the disk drive industries for years. Trace contaminants are not acceptable in the carbide, optics and ophthalmic industries, as they may cause adhesion failures in a multi-coating process that follows cleaning. For obvious reasons, absolutely clean surfaces are an extremely critical requirement in cleaning medical devices. Concern about particles has become a common denominator among all industries.
Precision Cleaning
Precision or critical cleaning of components or substrates is the complete removal of undesirable contaminants to a desired preset level. The preset level is normally the minimum level at which no adverse effects take place in a subsequent operation. To achieve this level, it is critical not to introduce new contaminant(s) into the cleaning process. For example, if the cleaning of organic and ionic contaminants is achieved by an aqueous process, it is important to have high quality water and the proper parameters in the rinsing stages. Otherwise, residual detergent and/or ionics from the rinsing water will be the new contaminants. If drying is slow, deionized rinse water may react with some metallic surfaces at high temperatures and create undesirable stains or marks. Re-contamination of cleaned parts with outgassed residues produced from packaging or storing materials is another
big concern.
To select an effective cleaning method, the three essential factors directly influencing cleaning results are the cleaning chemistry, the scrubbing method and the process parameters. The subject of examining various combinations of available cleaning methods and their effectiveness, or lack thereof, is massive and well-explained in the current literature (see references). The focus in this article will be on ultrasonic cavitations and the ultrasonic cleaning mechanism. Ultrasonic technology is proven to be a versatile method for cleaning various organic, inorganic and particle contaminants from various metallic and nonmetallic surfaces.
Ultrasonic Cavitations and Surface Cleaning
Cleaning with ultrasonics offers several advantages over other conventional methods. Ultrasonic waves generate and evenly distribute cavitation implosions in a liquid medium. The released energies reach and penetrate deep into crevices, blind holes and areas that are inaccessible to other cleaning methods. The removal of contaminants is consistent and uniform, regardless of the complexity and the geometry of the substrates. 
Ultrasonic waves are mechanical pressure waves formed by actuating the ultrasonic transducers with high frequency, high voltage current generated by electronic oscillators (power generators) (Figure 1). A typical industrial high power generator produces ultrasonic frequencies ranging from 20 - 120 kHz. Typical PZT transducers are normally mounted on the bottom and/or the sides of the cleaning tanks or immersed in the liquid. The generated ultrasonic waves propagate perpendicularly to the resonating surface. The waves interact with liquid media to generate cavitation implosions. High intensity ultrasonic waves create micro vapor/vacuum bubbles in the liquid medium, which grow to maximum sizes proportional to the applied ultrasonic frequency and then implode, releasing their energies. The higher the frequency, the smaller the cavitation size. The high intensity ultrasonics can also grow cavities to a maximum in the course of a single cycle. 
At 20 kHz the bubble size is roughly 170 microns in diameter (Figure 2). At a higher frequency of 68 kHz, the total time from nucleation to implosion is estimated to be about one third of that at 25 kHz. At different frequencies, the minimum amount of energy required to produce ultrasonic cavities must be above the cavitation threshold. In other words, the ultrasonic waves must have enough pressure amplitude to overcome the natural molecular bonding forces and the natural elasticity of the liquid medium in order to grow the cavities. For water, at ambient, the minimum amount of energy needed to be above the threshold was found to be about 0.3 and 0.5 watts/cm2 per the transducer radiating surface for 20 kHz and 40 kHz, respectively.
The energy released from an implosion in close vicinity to the surface collides with and fragments or disintegrates the contaminants, allowing the detergent or the cleaning solvent to displace it at a very fast rate. The implosion also produces dynamic pressure waves which carry the fragments away from the surface. The implosion is also accompanied by high speed micro streaming currents of the liquid molecules. The cumulative effect of millions of continuous tiny implosions in a liquid medium is what provides the necessary mechanical energy to break physically bonded contaminants, speed up the hydrolysis of chemically bonded ones and enhance the solubilization of ionic contaminants. The chemical composition of the medium is an important factor in speeding the removal rate of various contaminants.
Cavitation Generation and Abundance 
The ultrasonic cleaning model (Figure 3) illustrates the generating cavitations through at least three steps: nucleation, growth and violent collapse or implosion. The transient cavities (or vacuum bubbles or vapor voids), ranging 50-150 microns in diameter at 25 kHz, are produced during the sound waves' half cycles. During the rarefaction phase of the sound wave, the liquid molecules
are extended outward against and beyond the liquid natural physical elasticity/bonding/attraction forces, generating a vacuum nuclei that continue to grow. A violent collapse occurs during the compression phase of the wave. It is believed that the latter phase is augmented by the enthalpy of the medium and the degree of mobility of the molecules, as well as the hydrostatic pressure of the medium. Cavitations are generated in the order of microseconds. At the 20 kHz frequency, it is estimated that the pressure is about 35-70 K Pascal and the transient localized temperatures are about 5000癈, with the velocity of micro streaming around 400 Km/hr (Figure 2).
Several factors have great influence on the cavitation's intensity and abundance in a given medium. Among these factors are the ultrasonic wave form, its frequency and the power amplitude. Other determining factors are the colligative properties of the liquid medium, including viscosity, surface tension, density and vapor pressure; the medium temperature and the liquid flow, whether static or dynamic or laminar; and dissolved gases.
In general, at low frequencies (20-30 kHz), a relatively smaller number of cavitations with larger sizes and more energy are generated. At higher frequencies (60-100 kHz), much denser cavitations with moderate or lower energies are formed. Low frequencies are more appropriate for cleaning heavy and large-size components, while high frequency (60-80 kHz) ultrasonics is recommended for cleaning delicate surfaces and for the rinsing step.
For example, at 68 kHz, the cavitation abundance is high enough and mild enough to remove detergent films and remove submicron particles in the rinsing steps without inflicting damage on surfaces. The 35-45 kHz frequency range was found to be appropriate for a wide range of industrial components and materials.
Estimates of cavitation abundance at various ultrasonic frequencies have shown that the number of cavitation sites is directly proportional to the ultrasonic frequency. For example, about 60 to 70 percent more cavitation sites per unit volume of liquid are generated at 68 kHz than at 40 kHz. The average size of cavities is inversely proportional to the ultrasonic frequency.
Therefore, one would expect that at the higher frequency, at a given energy level, the scrubbing intensity would be milder, particularly on soft and thin or delicate surfaces, and more penetration and surface coverage into the recessed areas and small blind holes would be expected.
Ultrasonic Frequency and Particle Removal
Recent investigations have confirmed that higher frequencies are more effective for the removal of certain contaminants. Reports on particle removal efficiency have shown that the removal efficiency of one micron and submicron particles in deionized water has increased with the higher frequency. At 65 kHz, the removal efficiency of a one micron particle is 95 percent, versus 88 percent at 40 kHz. A similar increase in efficiency results was reported for 0.7 and 0.5 micron particles. It was also reported that there was zero or little difference in the removal efficiency of particles at the ultrasonic frequency of 65 kHz and at the megasonic frequency of 862 kHz. Both frequencies showed 95 percent removal efficiency of one micron particles and 87/90, 84/85 for 0.7 and 0.5 micron particles, respectively.
Aqueous and Semi-Aqueous Ultrasonic Cleaning
Cavitations generated in plain water can clean limited numbers of certain contaminants. However, cleaning is more complex in nature than just extracting the contaminants away from the surface. Consistency and reproducibility of results are the key, particularly in industrial production lines. Cleaning chemistry, as part of the overall cleaning process, is a crucial element in achieving the desired cleanliness. First, the selected chemistry must cavitate well with ultrasonics. Also, compatibility of the chemistry with the substrates, wettability, stability, soil loading, oil separation, effectiveness, dispersion of solid residues, free rinsability and chemistry disposal are all crucial issues that must be addressed when deciding on the proper chemistry. Chemistry is needed to do one or multiple tasks - to displace oils or solvents, to solubilize or emulsify organic contaminants, to encapsulate particles, to disperse and prevent redeposition of contaminants after cleaning. Special additives in cleaning chemistries can assist in the process of breaking chemical bonding, removal of oxides, preventing corrosion or enhancing the physical properties of the surfactants.
For example, we have found that ultrasonic cavitations enhanced the removal efficiency of hydrophobic solvent cleaning films by about 30 to 40 percent versus using a spray rinse technique, when coated metallic and nonmetallic surfaces were treated with aqueous displacement solutions (ADS). The ADS material is chemically designed to be compatible with the substrate and to rapidly displace hydrophobes. All tested surfaces were rendered solvent-free and hydrophilic.
Particles, in general, are not spherical and have irregular shapes. Some of the adhesive forces that influence detachment of a particle are van der Waals, electrical double layer, capillary and electrostatic. One would expect that small particles are easier to remove. The fact is that the smaller the particles, the more difficult they are to remove. The weight of a particle is another factor greatly influencing a particle detachment. Kaiser has recently reported that although the force between a particle and an adjacent surface decreases with particle size, it becomes more difficult to remove a solid particle from a solid surface because of the value of the ratio, Fa/W, where Fa is the force of attraction and W is the weight of the particle. The value of Fa/W increases rapidly as the diameter of a particle decreases.
Ultrasonic Systems
Typical ultrasonic aqueous batch cleaning equipment consists of four steps: ultrasonic cleaning, two ultrasonic reverse cascade water rinses and heated recirculated filtered clean air for drying. The number and the size of the stations are determined based on the required process time. A semi-aqueous cleaning system includes an extra station for solvent displacement, connected to a phase separation/recovery system. Typical tank size ranges from 20 liters to 2,000 liters, based on the size of the parts, production throughput and the required drying time. The cleaning process can be automated to include computerized transport systems able to run different processes for various parts simultaneously. The whole machine can be enclosed to provide a clean room environment meeting class 10,000 down to class 10 clean room specifications. Process control and monitoring equipment consists of flow controls, chemical feed-pumps, in-line particle count, TOC measurement, pH, turbidity, conductivity, refractive index, etc. The tanks are typically made of corrosion resistant stainless steel. However, other materials are also used - such as quartz, PVC, polypropylene or titanium - to construct tanks for special applications. Titanium nitride coating is used to extend the lifetime of the radiating surface in tanks or immersible transducers.
Automation of a batch cleaning system is an integral part of the system. Advantages of automation are numerous. Consistency, achieving throughputs, full control on process parameters, data acquisition and maintenance of process control records are just a few.
Mechanism of Cleaning
Two main steps take place in surface cleaning. The first step is contaminant removal and the second is keeping those contaminants from re-adhering to the surface. The removal of various contaminants involves different mechanisms, based on the nature and/or the class of the contaminant.
Three general classes of common contaminants are organic, inorganic and particulate matter. Particles do not necessarily belong to a certain class and can be from either class or a mixture. Contaminants of any class could be water soluble or water insoluble. Organic contaminants in most cases will be hydrophobic in nature, such as oils, greases, waxes, polymers, paints, print, adhesives or coatings.
Most inorganic materials are insoluble in solvents that are water-immiscible. Water is the best universal solvent for ionic materials, organics or inorganics. However, water insoluble inorganics, such as polishing compounds made of oxides of aluminum, cerium or zirconium, require a more complex cleaning system.
Organic contaminants can be classified into three general classes - long chain, medium chain and short chain molecules. The physical and chemical characteristics are related to their structure and geometry.
Organic contaminants are removed by two main mechanisms. The first is by solubilization in an organic solvent. Degree of solubilization in various solvents is directly related to their molecular structure. The second mechanism is by displacement with a surfactant film followed by encapsulation and
dispersion. 
In aqueous cleaning, the detergent contains surfactants as essential ingredients. Surfactants are long chain organic molecules with polar and non-polar sections in their chains. Surfactants can be ionic or non-ionic in nature, based on the type of functional groups attached to or part of their chains. When diluted with water, surfactants form aggregates called micelles (Figure 4) at a level above their critical micelle concentration (CMC). The circle in Figure 4 represents the ionic or the hydrophilic moiety and the line represents the nonionic or hydrophobic portion of the surfactant molecule. The equilibrium tends to shift to the right. 
The mechanism of removal of organic contaminants by detergent involves wetting of the contaminant as well as the substrate. According to Young's equation, this will result in increasing the contact angle between the contaminant and the surface, thus decreasing the surface area wetted with the hydrophobe, reducing the scrubbing energy for removal (Figure 5).
cos q=gSB - gSO
gOB
The ultrasonic cavitations play an important role in initiating and finishing the removal of such hydrophobic contaminants. The shock wave (the micro streaming currents) greatly speed up the breaking of the hanging contaminants, enhancing displacement with the detergent film. The removed contaminants are then encapsulated in the micellic aggregates, thus preventing their redeposition. The net result is that ultrasonic cavitations accelerate the displacement of contaminants from the surface of the substrate and also facilitate their dispersion throughout the cleaning system.
Particles, in general, have irregular shapes. All the adhesion forces - van der Waals, electrical double layer, capillary and electrostatic - in theory are directly proportional in magnitude to the size of the particle. One would expect that the energy of detachment would decrease with the size of particles. However, the smaller particles are always more difficult to detach. This is mainly due to the lodging effect. Smaller particles tend to get trapped in the valleys of a rough surface. 
The mechanism of particle removal involves shifting the free energy of detachment to be near or smaller than zero, according to Gibbs adsorption equation (Figure 6). Surfactants play a very important role in decreasing by adsorbtion at particle and substrate interfaces and with the bath.
D G=gSB + gOB - gOS
The wettability of the surface plays an important role in achieving this step. The ultrasonic cavitation's role is to provide the necessary agitation energy for the detachment (i.e. the removal force). At high frequency (60-70 kHz) ultrasonics, the detachment or the removal efficiency of one micron particles, measured in deionized water, was found to be 95 percent, equaling the efficiency obtained by using the megasonics at about 850 kHz, versus 88 percent at 40 kHz. This is expected in light of the fact that cavitation size is smaller at higher frequencies and can reach deeper into the surface valleys. One would then anticipate that by using a combination of the high frequency ultrasonics at 65-68 kHz and the appropriate chemistry, the removal efficiency of various particles can be further optimized.
Redeposition of Contaminants
Redeposition of contaminants is inhibited by another mechanism, by forming a barrier between the removed contaminant and the cleaned surface. In solvent cleaning, the adsorbed solvent layers on the substrate surface and the contaminants provide a film barrier. In aqueous cleaning, a good surfactant system is capable of encapsulating contaminants inside their micellic structure as depicted in Figure 7. Thus, redeposition of the encapsulated contaminants (soils) onto the surface is prevented via stearic hindrance for nonionic surfactants, while anionic surfactants prevent redeposition via electrical repulsive barrier. 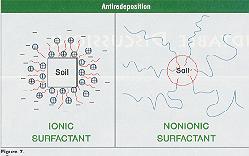
Encapsulation can be permanent or transient, based on the nature of the used surfactants. Transient encapsulation is superior to emulsification, as it allows better filtration and/or phase separation of contaminants. The potential of reversing the redeposition step by the sonic shock waves on loaded micelles results in partial re-adhesion. Therefore, allowing the increase in
the soil load in a cleaning solution to reach saturation point, without good filtration, will result in a significant decrease in the detergent cleaning efficiency, at which point the cleaning action may cease. To ensure steady cleaning efficiency, the dispersed con-taminants must be removed by means of continuous filtration or separation of contaminants, along with maintaining the recommended concentration of the cleaning chemical.
References
1. S.B. Awad, "An Ultrasonic Semi-Aqueous Alternative to Vapor Degreasing," Precision Cleaning, I (1), p. 75, 1993.
2. S.B. Awad, "Method for Cleaning and Drying of Metallic and Nonmetallic Surfaces," U.S. Patent, 5,397397, 1995.
3. A.A. Busnaina, G. W. Gale and I. I. Kashkoush, "Ultrasonic and Megasonic Theory and Experimentation," Precision Cleaning, 13, II (4), 1994.
4. D. Deal, "Coming Clean: What's Ahead in Silicon Wafer Cleaning Technology," Precision Cleaning, II (6), 24, 1994.
5. J.B. Durkee, The Parts Cleaning Handbook, Gardner Pub. Inc., Cincinnati, OH, 1994
6. EPA/400/1-91/016, "Aqueous and Semi-aqueous Alternatives For CFC-113 and Methyl Chloroform Cleaning of Printed Circuit Board Assemblies," 1991.
7. EPA/400/1-91/018, "Eliminating CFC-113 and Methylchloroform In Precision Cleaning Operations," 1991.
8. R. Kaiser, Particles on Surfaces 2, p. 269, K.L. Mittal, Editor, Plenum Press, NY, 1989.
9. R.C. Manchester, "Precision Aqueous Cleaning System and Process Design," Precision Cleaning, II (6), p. 11, 1994.
10. K.L. Mittal, Editor, Particles on Surfaces 3, Plenum Press, New York, 1991.
11. K.L. Mittal, "Surface Contamination Concepts and Concerns," Precision Cleaning, III (1), p. 17, 1995.
12. R. Naggarian, R. W. Welker and R. L. Weaver, "Evaluation of Aqueous Cleaning for Disk Drive Parts," Microcontamination Proceedings, p. 113, 1990.
13. M. O'Donoghue, "The Ultrasonic Cleaning Process," Microcontamination, 2 (5), 1984.
14. S.S. Seelig, "The Chemical Aspects of Cleaning," Precision Cleaning, III (10), p. 33, 1995.
15. K.S. Suslick, Editor, Ultrasound, Its Chemical, Physical and Biological Effects, VCH Publishers, Inc., 1988.
16. K.S. Suslick, "The Chemical Effects of Ultrasound," Sci. Amer., 80, 1989.
About the Author
Dr. Sami Awad is VP of technology at Crest Ultrasonics (Trenton, NJ). He has more than 15 years experience in developing new chemistries and processes for precision cleaning, surface treatment and metal forming. Awad is the author of more than 25 scientific academic papers in organic synthesis and reaction mechanisms, and has served on the teaching faculties of Drexel University (Philadelphia, PA) and Cairo University, Egypt. Awad is a member of ACS, Ultrasonic Industrial Association (UIA), IDEMA and ASM. He holds a Ph.D. in organic chemistry, and may be reached at (609) 883-4000 or by fax at (609) 883-3331. |